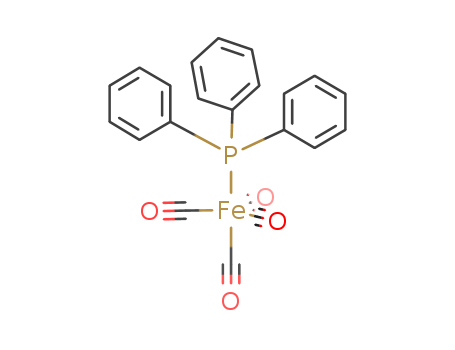
(Benzylideneacetone)iron tricarbonyl is the organoiron compound with the formula (C6H5CH=CHC(O)CH3)Fe(CO)3. It is a reagent for transferring the Fe(CO)3 unit. This red-colored compound is commonly abbreviated (bda)Fe(CO)3.
Structure and bonding
(bda)Fe(CO)3 is an example of a complex of an η2-ketone. It is a piano stool complex. The compound is characterized by IR bands at 2065, 2005, and 1985 cm−1 (cyclohexane solution), the three bands being indicative of the low symmetry of the complex, which is chiral.
Synthesis, reactions, related reagents
It is prepared by the reaction of Fe2(CO)9 with benzylideneacetone.
(bda)Fe(CO)3 sometimes reacts with Lewis bases to give adducts without displacement of the bda. The reagents of the type (bda)Fe(CO)2(PR3) function as sources of "Fe(CO)2(PR3)" (R = aryl, etc.).
Other sources of Fe(CO)3 are Fe2(CO)9 and Fe(CO)3(cyclooctene)2. The latter is highly reactive and thermally sensitive. Imine derivatives of cinnamaldehyde, e.g. C6H5CH=CHC(H)=NC6H5, also form reactive Fe(CO)3 adducts, which have been shown to be superior in some ways to (bda)Fe(CO)3.
References
Further reading
- Alcock, Nathaniel W.; Richards, Christopher J.; Thomas, Susan E. (1991). "Preparation of tricarbonyl(.eta.4-vinylketene)iron(0) complexes from tricarbonyl(.eta.4-vinyl ketone)iron(0) complexes and their subsequent conversion to tricarbonyl(.eta.4-vinylketenimine)iron(0) complexes". Organometallics. 10 (1): 231–238. doi:10.1021/om00047a054. ISSN 0276-7333.